
write the balanced equation, including all physical states.ġ. predict the names and states of the products formed predict the reaction type (precipitation, neutralization or gaseous) - record your observations When finished, complete the data sheet by writing the balanced equation for each reaction. Aqueous iron (III) chloride + aqueous ammonium hydroxide.Aqueous sodium chloride + aqueous potassium nitrate.Aqueous sodium carbonate + aqueous cobalt (II) nitrate.Hydrochloric acid + aqueous sodium hydroxide.Aqueous barium chloride + sulfuric acid.Aqueous nickel (II) nitrate + aqueous sodium hydroxide.Hydrochloric acid + solid sodium bicarbonate (just a small scoop).Aqueous sodium phosphate + aqueous copper (II) sulfate.Aqueous sodium chloride + aqueous silver nitrate.

All waste is to be disposed of in the plastic container in the hood! If results are not obtained immediately, give the reaction some time.

Perform the following reactions, and record your observations for each on the data sheet.
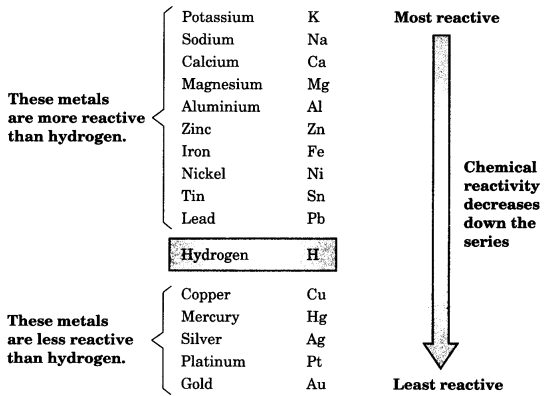
#CHEMLAB 10 ACTIVITIES OF METALS FULL#
A good estimate is to use three full dropper squirts of each chemical. Use approximately 3 mL quantities of all solutions. Most phosphates, carbonates, chromates and sulfides are insoluble (except those of the alkali metals and ammonium).Most sulfates are soluble, except for BaSO 4, SrSO 4, Ag2SO 4, PbSO 4, and CaSO 4.All halides (chlorides etc.) are soluble except for those containing Ag +1, Pb +2, and Hg 2 +2.Hydroxides of alkali metals and NH 4 +1, Ca +2, Sr +2, and Ba +2 are soluble.Alkali metal compounds, acetates, nitrates, and ammonium compounds are all soluble.Always name the cation first, then the anion.Įxample 6: Name the ionic compound Al(NO 3) 3.Ĭation = Al +3 = aluminum anion = NO 3 -1 = nitrate The name of this compound is aluminum nitrate Both the cation and anion must be named.Ģ. The basic rules for writing names of ionic compounds: 1. The numbers of each element present in the compound become subscripts in the chemical formula.Įxample 5: Write the formula for magnesium phosphate.įirst identify the cation and anion in this compound.Ĭation = magnesium = Mg +2 anion = phosphate = PO 4 -3įor a neutral compound, three Mg +2 are needed for every 2 PO 4 -3 The formula of the compound is Mg3(PO4)2 Total charge of all the positive cations must equal the total charge of all the negative anions in the compound.



 0 kommentar(er)
0 kommentar(er)
